

CPAP Machine Recall
Have you or a loved one suffered injuries related to the use of a Philips CPAP Sleep Apnea Machine?
Request A Free Legal Case Review


Injuries and Health Risks of Sleep Apnea Machines
Philips Respironics has received multiple complaints from it’s customers about black debris or particles in the airpath circuit of their mechanical ventilators, BiPAP, and CPAP devices, though the cause of the symptoms has not been definitively linked at this time.
Potential risks associated with the recalled Philips sleep apnea devices can be life-threatening and include:
- Asthma
- Carcinogenic effects (particularly to the kidneys, liver, and lungs)
- Cough and chest pressure
- Eye, nose, skin, and respiratory tract irritation
- Headaches or dizziness
- Hypersensitivity
- Inflammatory response
- Nausea and vomiting
- Organ damage
- Sinus infection
Philips Recalls Millions of CPAP and Other Breathing Machines
The voluntary recall announcement issued in June 2021 is a follow-up to a previous notification from April 2021. The recall involves millions of sleep apnea and ventilator devices which may increase the risk of lung injury and cancer due to inhalation foam particles and outgassing of toxic chemicals. Philips estimates that 3 to 4 million devices may be affected, the majority of which are first-generation DreamStation products sold before April 2021.
Here Is a list of Recalled Philips Devices:
CPAP and BiLevel PAP
- Continuous Ventilator, Non-life Supporting
- DreamStation ASV
- DreamStation ST, AVAPS
- SystemOne ASV4
- C Series ASV, S/T, AVAPS
- OmniLab Advanced Plus In-Lab Titration Device
- Non-continuous Ventilator
- SystemOne Q series
- DreamStation CPAP, Auto CPAP, BiPAP
- DreamStation Go CPAP, APAP
- Dorma 400, 500 CPAP
- REMStar SE Auto CPAP
- Continuous Ventilator, Minimum Ventilatory Support, Facility Use
- E30 (Under Emergency Use Authorization)
Mechanical Ventilators
- Continuous Ventilator
- Trilogy 100 Ventilator
- Trilogy 200 Ventilator
- Garbin Plus, Aeris, LifeVent Ventilator
- Continuous Ventilator, Minimum Ventilatory Support, Facility Use
- A-Series BiPAP V30 Auto Ventilator
Sound-reducing Foam Liner May Be to Blame
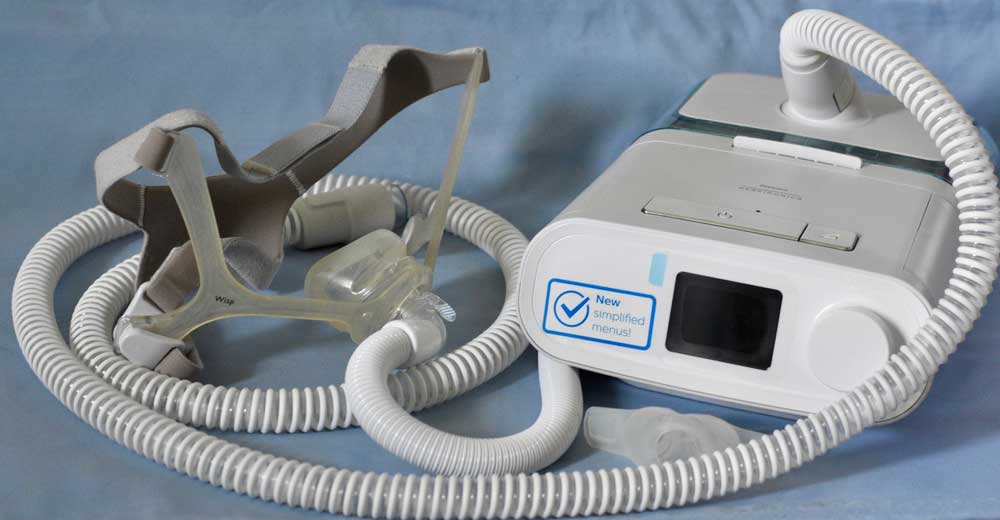
The recalled Philips CPAP and ventilator devices have been linked to an increased risk of airway contamination, chemical exposure, and possible cancer. The problem is related to polyester-based polyurethane (PE-PUR) foam which is a sound-abatement component of certain models of the devices.

Tell us more about your case.
888-888-8888
CLICK TO CALL
Disclaimer: Past results afford no guarantee of future results and each case is different and is judged on its own merits. Some cases result in no recovery. Costs and expenses will be advanced and reimbursed to us only if you recover. You have no liability for costs or expenses unless a court directs. The choice of a lawyer is an important decision and should not be based solely upon advertisements. Some matters may be referred to other lawyers. The choice of a lawyer is an important decision and should not be based solely upon advertisements. Never stop taking any prescription drug without first consulting with a doctor. The information on this website is for general information purposes only. Nothing on this site should be taken as legal advice for any individual case or situation. This information is not intended to create, and receipt or viewing does not constitute, an attorney-client relationship. Some online reviews may have been given compensation for testimony or endorsement. Results are not guaranteed and that past performance is not an indication of future success.